Per- and polyfluoroalkyl substances (PFAS)—often called “forever chemicals”—are among the most stubborn contaminants found in drinking water today. Designed to resist heat, water, and degradation, these synthetic compounds persist in the environment and accumulate in the human body, making them notoriously difficult to remove using conventional treatment methods.
In a new study, researchers from the University of California Berkeley, the Colorado School of Mines, and Konkuk University in Seoul, and report a promising new approach: a family of porous polymer materials designed to rapidly and efficiently capture PFAS from water.
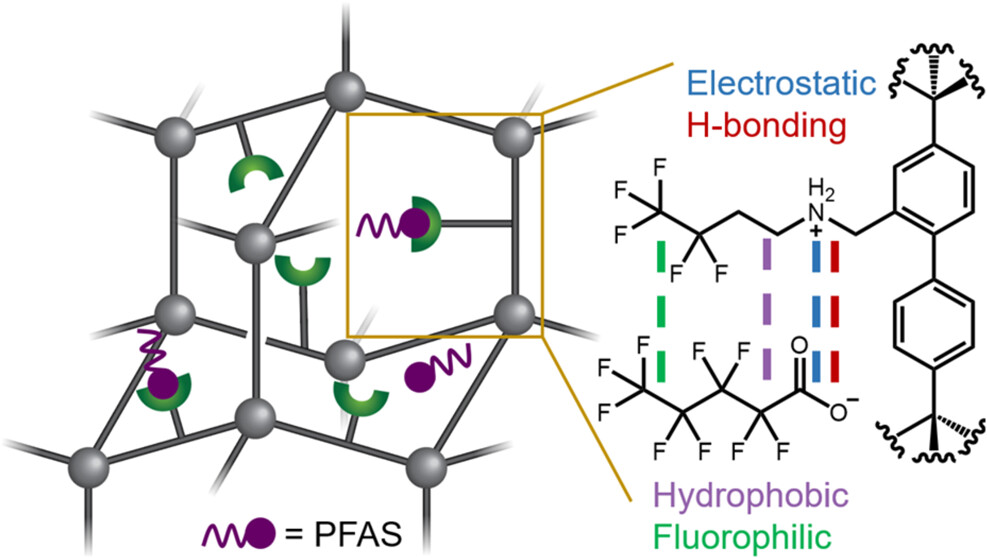
Rather than relying on a single material, the team developed a library of sponge-like adsorbents, each engineered with distinct chemical features intended to attract PFAS molecules. As contaminated water flows through the adsorbents, PFAS compounds bind to the material while clean water passes through. Testing the materials side by side allowed the researchers to directly compare how different chemical interactions embedded within the materials influence PFAS capture under realistic water conditions.
One clear trend emerged. Materials containing a positive charge were especially effective at drawing PFAS molecules in, highlighting electrostatic attraction as a key design principle for future PFAS adsorbents. Among the materials tested, one stood out for its performance—though the researchers emphasize that effectiveness alone is not enough.
The study also addresses a critical, and often overlooked, question in PFAS remediation: what happens after PFAS are removed from water? Captured PFAS must still be managed safely to avoid simply shifting contamination from one place to another. The researchers explore strategies for concentrating recovered PFAS so they can be more efficiently destroyed using emerging treatment technologies.
By considering adsorption and material regeneration together, this work underscores the importance of PFAS treatment solutions that function across the entire treatment lifecycle. Beyond demonstrating strong performance, the study provides practical design guidance for developing next-generation materials that are safer, more effective, and better suited for real-world water treatment systems.
As communities continue to grapple with widespread PFAS contamination, this research represents an important step toward technologies capable of addressing not just the presence of PFAS—but the full challenge of removing and ultimately eliminating them from water supplies.
